Description
Summary:
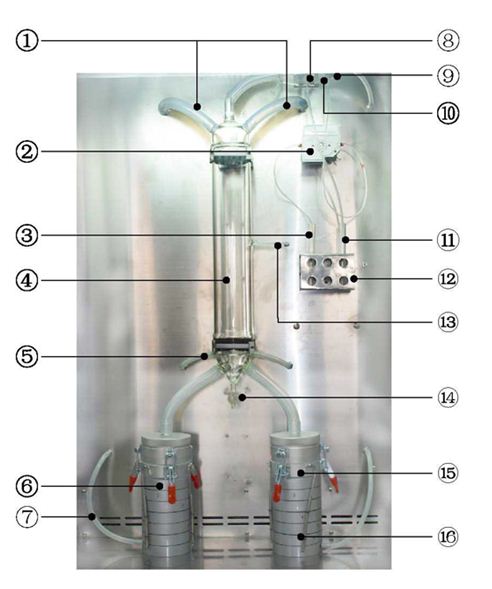
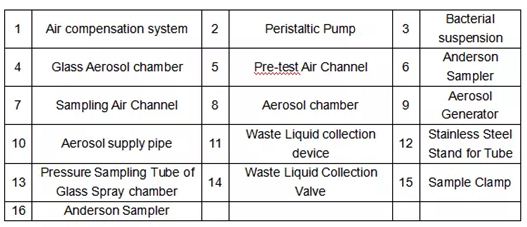
This Mask Bacterial Filtration Efficiency Tester is used to test the percentage of respirator material to remove bacteria-containing suspended particles at a specified flow rate. The method of double air sampling is adopted to improve the accuracy of sampling, which is suitable for the performance test of bacterial filtration efficiency of surgical masks by metrological verification departments, scientific research institutes, medical mask manufacturers and other relevant.
Face Masks bacterial filtration efficiency BFE tester is to determine the bacterial filtration efficiency (BFE) of medical face mask materials by employing a ratio of the upstream bacterial challenge to downstream residual concentration of the tested medical face mask materials. The tester provides certain flow rate of bacterial aerosol. The operator can measure the number of colony forming units passing through the medical face mask material, which is clamped between a six-stage Anderson cascade impactor and an aerosol chamber, expressed as a percentage of the number of colony forming units present in the challenge aerosol.
Standards:
YY / T 0469-2011: Medical surgical mask 5.6.2 Particle filtration efficiency.
YYT0969-201: Single-use Medical Face Mask
EN 14683: Medical Face Mask – Requirements and Test methods
ASTM F 2100: Standard Specification for Performance of Materials Used in Medical Face Masks
ASTM F 2101: Standard Test Method for Evaluating the Bacterial Filtration Efficiency (BFE) of Medical Face Mask Materials, Using a Biological Aerosol of Staphylococcus aureus
CNS 14775: Method of test for evaluating the bacterial filtration efficiency (BFE) of medical face mask materials, using a biological aerosol of staphylococcus aureus.
Features:
* Professional negative pressure bio-safety cabinet technology to ensure the safety of operators and the surroundings;
* High negative pressure working chamber, two-stage high efficiency filter, 100% safe discharge;
* Two – way, six-stage Anderson accurate sampling is adapted.
* Built-in peristaltic pump and the volume is adjustable.
* Special microorganism aerosol generator with adjustable bacteria flow and good performance of atomization.
* Separated and color touch screen control, easier to operate.
* USB interface, can reserve your test data in different forms.
* Equipped leakage protection and operating window provide better operating view and safety operating
conditions.
* Stainless steel chamber with insulation and fire retardant lining is stable and reliable.
Main Parameters:
| Item | Parameter Range | Resolution | Max Permissible Errors |
| Sampling flow rate of Path A | 28.3L/min | 0.1L/min | ±2.5% |
| Sampling flow rate of Path B | 28.3L/min | 0.1L/min | ±2.5% |
| Nebulization flow rate | (8~10)L/min | 0.1L/min | ±5.0% |
| Peristaltic pump flow rate | 0.006~3mL/min | 0.001mL/min | ±2.5% |
| Flow meter fore pressure of path A | -20~0kPa | 0.01kPa | ±2.5% |
| Flow meter fore pressure of path B | -20~0kPa | 0.01kPa | ±2.5% |
| Nebulization flow meter fore pressure | 0~300kPa | 0.1kPa | ±2.5% |
| Negative pressure of aerosol chamber | -90~-120Pa | 0.1Pa | ±2.0% |
| Negative pressure of cabinet body | (-50~-200)Pa | ||
| Data Storage | >100000 Groups | ||
| Feature of HEPA filter | The filtration efficiency of particles above 0.3um is ≥99.99% | ||
| Mass mean diameter of aerosol generator | Mean particle size (MPS) (3.0±0.3)μm, geometric std. deviation≤1.5 | ||
| Andersen |
I>7μm, II. 4.7-7μm, III. 3.3-4.7μm, IV. 2.1-3.3μm, V. 1.1-2.1μm VI. 0.6-1.1μm |
||
| Size of aerosol chamber | 600mm(Length)×85mm(Diameter)×3mm(Thickness) | ||
| Total particle number of positive control sampler | 2200±500cfu | ||
| Ventilation flow of negative pressure cabinet | ≥5m3/min | ||
| Negative pressure cabinet door size | 1000mm×730mm (L×W) | ||
| Dimension of main machine | 1180mm×650mm×1300mm (L×W×H) | ||
| Dimension of stand | 1180mm×650mm×600mm (L×W×H), height adjustable within 100mm | ||
| Power supply | AC220V±10%,50Hz | ||
| Noise | <65dB(A) | ||
| Weight | Approx. 180kg | ||
| Power | <1500W | ||













Reviews
There are no reviews yet.